Clinical Studies
TRIUMEQ* Was Generally Better Tolerated vs Atripla® up to 144 Weeks With Fewer Discontinuations2
Discontinuations due to adverse events
SINGLE—Proportion of patients with adverse events leading to discontinuation at 48, 96, and 144 weeks2,3
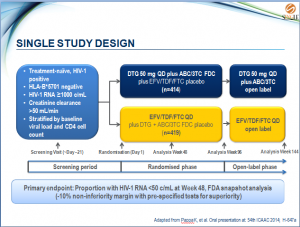
- Treatment-naïve, HIV-1 positive
- HLA-B*5701 negative
- HIV-1 RNA ≥1000 c/mL
- Creatinine clearance
>50 mL/min - Stratified by baseline viral load and CD4 cell count
- Primary endpoint: Proportion with HIV-1 RNA <50 c/mL at Week 48, FDA snapshot analysis
(-10% non-inferiority margin with pre-specified tests for superiority)
- Treatment-naïve, HIV-1 positive
- HLA-B*5701 negative
- HIV-1 RNA ≥1000 c/mL
- Creatinine clearance
>50 mL/min - Stratified by baseline viral load and CD4 cell count
- Treatment-naïve, HIV-1 positive
- HLA-B*5701 negative
- HIV-1 RNA ≥1000 c/mL
- Creatinine clearance
>50 mL/min - Stratified by baseline viral load and CD4 cell count
DTG + ABC/3TC had statistically superior efficacy vs EFV/TDF/FTC
88% vs 81% were undetectable at 48 weeks (P=0.003)
80% vs 72% remained subsequently undetectable at 96 weeks (P=0.006)
71% vs 63% remained subsequently undetectable at 144 weeks (P=0.010)
DTG + ABC/3TC is effective regardless of baseline viral load
83% of treatment-naïve patients with HIV-1 RNA >100,000 copies/mL remained undetectable at 48 weeks
DTG + ABC/3TC was still as effective as EFV/TDF/FTC in patients with high baseline viral loads at 96 weeks
69% of treatment-naïve patients with HIV-1 RNA >100,000 copies/mL remained undetectable at 144 weeks
DTG + ABC/3TC was generally better tolerated vs EFV/TDF/FTC with fewer discontinuations
2% vs 10% discontinued due to AEs at 48 weeks
3% vs 11% discontinued due to AEs at 96 weeks
4% vs 14% discontinued due to AEs at 144 weeks
No INI or NRTI resistance up to 144 weeks with DTG + ABC/3TC
- Treatment-naïve, HIV-1 positive
- HLA-B*5701 negative
- HIV-1 RNA ≥1000 c/mL
- Creatinine clearance
>50 mL/min - Stratified by baseline viral load and CD4 cell count
- Primary endpoint: Proportion with HIV-1 RNA <50 c/mL at Week 48, FDA snapshot analysis
(-10% non-inferiority margin with pre-specified tests for superiority)
- DTG + ABC/3TC had statistically superior efficacy vs EFV/TDF/FTC88% vs 81% were undetectable at 48 weeks (P=0.003)80% vs 72% remained subsequently undetectable at 96 weeks (P=0.006)71% vs 63% remained subsequently undetectable at 144 weeks (P=0.010)
DTG + ABC/3TC is effective regardless of baseline viral load
83% of treatment-naïve patients with HIV-1 RNA >100,000 copies/mL remained undetectable at 48 weeks
DTG + ABC/3TC was still as effective as EFV/TDF/FTC in patients with high baseline viral loads at 96 weeks
69% of treatment-naïve patients with HIV-1 RNA >100,000 copies/mL remained undetectable at 144 weeks
DTG + ABC/3TC was generally better tolerated vs EFV/TDF/FTC with fewer discontinuations
2% vs 10% discontinued due to AEs at 48 weeks
3% vs 11% discontinued due to AEs at 96 weeks
4% vs 14% discontinued due to AEs at 144 weeks
No INI or NRTI resistance up to 144 weeks with DTG + ABC/3TC
- Treatment-naïve, HIV-1 positive
- HLA-B*5701 negative
- HIV-1 RNA ≥1000 c/mL
- Creatinine clearance
>50 mL/min - Stratified by baseline viral load and CD4 cell count
- Treatment-naïve, HIV-1 positive
- HLA-B*5701 negative
- HIV-1 RNA ≥1000 c/mL
- Creatinine clearance
>50 mL/min - Stratified by baseline viral load and CD4 cell count